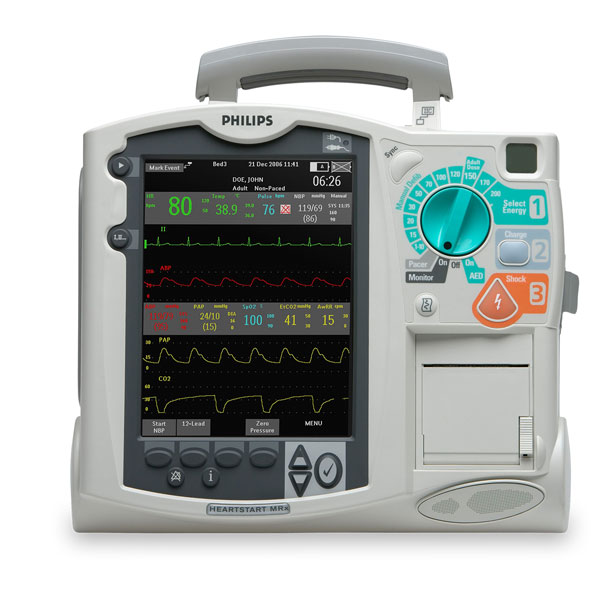
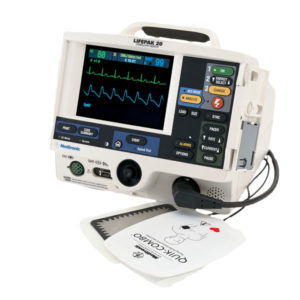
Philips HeartStart MRX
Certified Refurbished Medical Equipment!
The Philips HeartStart MRx has flexible, fast, and reliable 12-lead transmission capabilities so you can send data using your choice of technologies to wherever you need it to go – ED, Cath Lab, cardiologist’s smartphone – to begin the next level of care. Only Philips has the advanced DXL 12-Lead ECG algorithm, which takes STEMI clinical decision support to a new level with the unique capabilities of the Philips HeartStart MRx that enable confident decision-making to help speed triage.
$3,318.35
Philips HeartStart Mrx Features
The Philips HeartStart MRx has flexible, fast, and reliable 12-lead transmission capabilities so you can send data using your choice of technologies to wherever you need it to go – ED, Cath Lab, cardiologist’s smartphone – to begin the next level of care. Only Philips has the advanced DXL 12-Lead ECG algorithm, which takes STEMI clinical decision support to a new level with the unique capabilities of the Philips HeartStart MRx that enable confident decision-making to help speed triage.
Philips Heartstart Mrx for Hospitals –
Ease of use is the hallmark of all Philips defibrillators and the HeartStart MRx is no exception. In the moment of need, the MRx’s intuitive and easy-to-use design can help your team save a life.
Clinical networking capability on the IntelliVue Clinical Network for monitoring and review at the central station. MRx is the only monitor/defibrillator that has the ability to connect to a clinical monitoring network.
Scalable platform for use as a crash cart or as a high-end critical care transport monitor, the MRx can be easily upgraded in-house so that you can receive the benefits of Philips advancements now and into the future.
Standardized alarms, cables, and interchangeable accessories among Philips defibrillators and IntelliVue patient monitors mean enhanced ease of use and “plug and play” patient hand-off, as well as simplified inventory management and reduced costs.
Additional Features:
The Philips Heartstart Mrx is an adjustable ECG size and autogain
8.4 inch (diagonal), 4-wave color display, largest in its class.
12-Lead data transmission
Data collection and event summary.
Drager Apollo Specifications
Dimensions (Without External Paddles)
Width: 12.4 in (31.3 cm)
Depth: 8.3 in (21 cm)
Height: 11.7 in (29.5 cm)
Dimensions (With External Paddles)
Width: 13.4 in (34 cm)
Depth: 8.3 in (21 cm)
Height: 13.6 in (34.5 cm)
Weight
13.2 lbs. (6kg): Base unit with 1 battery, pads, and pads cable.
+4.1 lbs. (1.86 kg): With added carrying case.
+2.5 lbs. (1.1 kg): With added paddle tray and external standard paddles.
Environmental
Water Resistance: Meets IEC 60601-2-4
Solids Resistance: IP2X
Temperature
Operating: 32º – 113º F (0º – 45º C)
Storage: -4º – 158º F (-20º – 70º C)
Humidity: Operating: 0% to 95% relative
Safety: Meets EN 60601-1, UL 2601-1, CSA C22.2 No. 601-1-M90 CSA, EN 60601-2-4
Display
Dimensions: 8.4 in diagonal (12.8 cm x 17.1 cm)
Type: TFT color LCD
Resolution: 640 x 480 pixels (VGA)
Wave Viewing Time: 5 seconds (ECG)
Strip Chart Printer
Printer
Standard: 50 mm (paper width) thermal array printer.
Optional: 75 mm (paper width) thermal array printer.
Continuous ECG Strip: Prints primary ECG lead with event annotations and measurements in real-time or with a 10-second delay.
Auto Printing: Printer can be configured to print marked events, charge, shock, and alarms.
Reports: Event Summary, 12-Lead, Vital Signs Trending, Operational Check, Configuration, Status Log, and Device Information.
Paper Size
1.97 in (50 mm) W by 100 ft. (30 m) L.
2.95 in (75 mm) W by 100 ft. (30 m) L.
Defibrillator
Model: Philips HeartStart MRx (M3535A)
Waveform: Biphasic Truncated Exponential. Waveform parameters adjusted as a function of patient impedance.
Output Energy: Manual (selected): 1-10, 15, 20, 30, 50, 70, 100, 120, 150, 170, 200 Joules maximum energy, limited to 50 Joules for internal defibrillation. AED Mode (single energy output): 150 Joules into a 50-ohm load.
Charge Time: Less than 5 seconds to 200 Joules with a new, fully charged lithium-ion battery at 25º C.
Shock Delivery: Via multifunction defib electrode pads or paddles.
Quick Shock: Less than 10 seconds from the cessation of CPR to shock delivery.
Patient Impedance Range
Minimum: 15 ohm (internal defibrillation); 25 ohm (external defibrillation).
Maximum: 150 ohm.
AED Mode: Shock advisory sensitivity and specificity meet AAMI DF-39 guidelines.
Battery
Type: 6.0 Ah, 14.8 V, rechargeable lithium-ion.
Dimensions
Height: 6.5 in (165 mm)
Width: 3.8 in (95 mm)
Depth: 1.6 in (42 mm)
Weight: 1.6 lbs. (0.73 kg)
Charge Time: Approximately 3 hours to 100%, 2 hours to 80%.
Capacity
At least 5 hours of monitoring with ECG, SpO2, CO2, temperature and two invasive pressures monitored continuously, NBP measured every 15 minutes, and 20 200J discharges (with a new, fully charged battery, operating at room temperature, 25º C).
At least 3.5 hours of monitoring with ECG, SpO2, CO2, temperature and two invasive pressures monitored continuously, NBP measured every 15 minutes and pacing at 180ppm at 160mA.
Battery Indicators
Battery gauge on battery, a capacity indicator on display; flashing RFU indicator, chirp, and ‘Low Battery’ message appears on display for low battery condition, when 10 minutes of monitoring time and 6 maximum energy discharges remain (with a new battery at room temperature, 25º C).
Data Storage
Internal: 12 hours of continuous ECG waveforms and events, maximum capacity of 55 event summaries.
Data Card: 60 event summary reports or 240 megabytes of patient data.
ECG and Arrhythmia Monitoring
Input
Up to 4 ECG waves displayed and up to 2 ECG waves print simultaneously.
Lead I, II, or III obtained through 3-lead ECG cable and separate monitoring electrodes. With 5-lead cable, obtain leads aVR, aVL, aVF or V. Pads ECG obtained through 2 multifunction defibrillation electrode pads.
Lead Fault: ‘Lead Off’ message and dashed line displayed, if an electrode or lead wire becomes disconnected.
Pads Fault: Dashed line displayed if a pad becomes disconnected.
Heart Rate Display: Digital readout on display 15 to 300 bpm, accuracy ±10%.
Heart Rate Arrhythmia Alarms: HR, Asystole, VFIB/VTACH, VTACH, extreme tachycardia, extreme bradycardia, PVC rate, Pacer not capture, Pacer not pacing.
ECG Size: 2.5, 5, 10, 20, 40 mm/mV, autogain.
Available Options
Non-Invasive Pacing: SpO2 pulse oximetry.
Non-Invasive Blood pressure: CO2 monitoring.
Invasive Blood Pressure (2 lines): Continuous temperature monitoring.
12-Lead Acquisition: 12-lead transmission.
Q-CPR Measurement and Feedback: Audio recording.
ACI-TIPI & TPI Predictive Instruments: Periodic clinical data transmission.
Batch/LAN Data Transfer.